MammaPrint is supported by the highest level of clinical evidence (level 1A) from MINDACT,1 a landmark independent trial published in the New England Journal of Medicine in 2016.
MINDACT stands for Microarray In Node-negative (or 1-3 positive lymph node) Disease may Avoid ChemoTherapy. It was a phase III, prospective, randomized, clinical study for a breast cancer recurrence test.
The trial investigated the clinical utility of MammaPrint, when compared to (or used along with) standard pathological criteria for the selection of patients unlikely to benefit from adjuvant chemotherapy.
Participants were categorized as low or high risk for cancer recurrence in two ways: first, through analysis of tumor tissue using MammaPrint; and second, using Adjuvant! Online, a tool which calculates risk of breast cancer recurrence based on common clinicopathological factors.
Patients characterized as low risk with both clinical and genomic assessments were spared chemotherapy, while patients characterized as high risk with both were advised to undergo chemotherapy. Those with discordant results were randomized to use either clinical or genomic (MammaPrint) risk evaluation to decide on chemotherapy treatment.
MINDACT Findings

of MINDACT clinically high risk patients were reclassified as genomically low risk by MammaPrint and therefore could be spared adjuvant chemotherapy
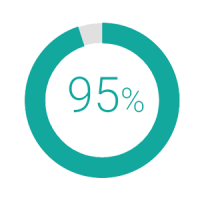
for clinically high risk patients who were reclassified by MammaPrint as genomically low risk, 95% were free of distant metastasis* at 5 years without chemotherapy

for patients who were high clinical risk but MammaPrint low risk with 1-3 involved lymph nodes, 96% were free of distant metastasis* at 5 years without chemotherapy
*The time to appearance of distant metastasis. A distant metastasis refers to cancer that has spread from the original tumor to distant organs.
†Prospective refers to when patients are recruited to a clinical study and followed forward in time.
‡Randomization is a procedure to allocate clinical study participants into different groups. By using chance to allocate participants, the groups are likely to be similar and allow the effects of the treatments to be compared more fairly.
REFERENCES
1 Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med. 2016; 375(8):717-29

