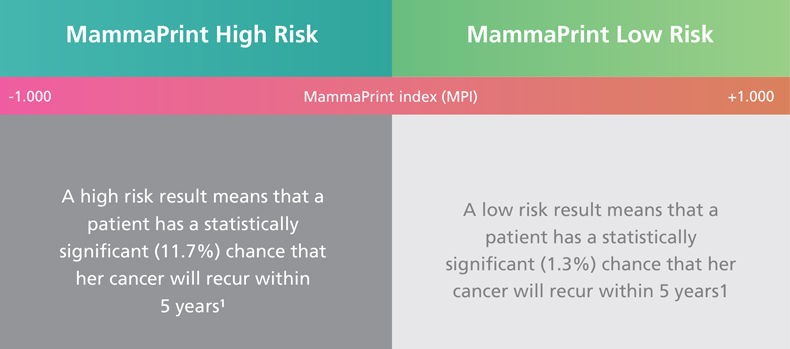
MammaPrint
MammaPrint analyzes the 70 most important genes associated with breast cancer recurrence to classify each breast cancer patient into “Low Risk” or “High Risk” of developing metastases within the first 10 years after diagnosis—without the ambiguity associated with “intermediate” scores found in some other tests.
The clarity that MammaPrint delivers helps patients and physicians make informed, confident treatment management decisions.
MammaPrint is FDA-cleared (US) for women of all ages. The device is also CE marked allowing for use in the European Union.
The test is validated by peer-reviewed data and supported by the highest level of clinical evidence from a landmark randomized,* prospective,† phase III‡ clinical trial known as MINDACT.

Recommended by expert medical guidelines
MammaPrint is included in numerous clinical practice guidelines developed by world-recognized cancer care organizations. Clinical guidelines are evidence-based recommendations for healthcare professionals involved in the management of patients.
Guidelines aim to improve the quality of care and services based on the most up to date peer-reviewed evidence. For example, MammaPrint is the only test of its kind endorsed by the American Society of Clinical Oncology for lymph node-positive patients.







*Randomization is a procedure to allocate clinical study participants into different groups. By using chance to allocate participants, the groups are likely to be similar and allow the effects of the treatments to be compared more fairly.
†When patients are recruited to a clinical study and followed forward in time.
‡A type of clinical trial designed to confirm and expand on the safety and/or effectiveness of an intervention from earlier clinical trials. Phase III trials are often large and may consist of hundreds or even thousands of patients.
REFERENCE
2 Wuerstlein R, et al. Results of multigene assay (MammaPrint®) and molecular subtyping (BluePrint®) substantially impact treatment decision making in early breast cancer: Final analysis of the WSG PRIMe Decision Impact Study. Poster presented at San Antonio Breast Cancer Symposium. December 2016; San Antonio, Texas